Abstract
The present study deals with the assessment of radon (Rn-222) concentration in groundwater and associated radiation doses among the local inhabitants due to its exposure in different wards of Purulia municipality of West Bengal, India. Radon concentrations in 120 groundwater samples collected from the municipal area have been measured using AlphaGuard radon monitor, and the value has been found to vary between 10.44 Bq/l and 403.56 Bq/l. The annual effective doses due to inhalation and ingestion of groundwater radon have been estimated for adults, children and infants, and the average doses for all three types have been found to be well above the reference dose level (RDL) of 0.1 mSv/y proposed by the World Health Organization (WHO). Additionally, some major cations (Na+, K+, Ca2+, Li+) and pH of the water samples have been analysed with an aim to find possible correlation between these parameters and water radon concentration. Low to moderate correlations have been observed between radon and these parameters. However, the high doses associated with inhalation and ingestion of radon suggest that there may be some potential health hazard risks among the local population which attracts immense importance towards studying radiation protection in this area.
Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.Avoid common mistakes on your manuscript.
Introduction
Radon, the only natural radioactive gas, which is produced from the α-decay of radium-226, is part of the uranium-238 series (Cothern 2014). The half-life of radon is 3.82 days, and this is long enough for diffusive transport through the fissures in rocks and dissolution into ground water due to its appreciable solubility in water (0.01 mol kg−1 bar−1 at 293 K) (Rangaswamy et al. 2016; Lima-Flores et al. 2021). High radon content in groundwater is subject to the presence of shear zones and other geological features, and the presence of granite bedrocks—containing uranium and radium—through which the water passes (Singh et al. 2008; Kareem et al. 2020; Abdallah et al. 2007). In addition, abundance of radon in groundwater depends on the porosity, permeability and the moisture content in the sub-surface (Jaishi et al. 2014; Günay et al. 2019). The dissolved radon can escape into the atmosphere by diffusion (Kessongo et al. 2020). Average transfer coefficient from water to air for radon is 10−4 (National Research Council 1999). This indicates that the concentration of most of the indoor radon gas comes from the household usage of radon-rich water. Rn-222 has two progeny, namely, Po-214 having half-life of 160 µs and Po-218 having half-life of 3 min. They are responsible for the majority of the radioactivity resulting from radon gas.
Approximately half of total radiation dose received by the population globally comes from the inhalation or ingestion of radon (UNSCEAR 2000; Naskar et al. 2018). The alpha particles emitted from radon can enter into the lungs of human being by inhalation of radon-containing air, which may lead to lung cancer (Kessongo et al. 2020; Agoubi 2021).When groundwater with high radon concentration is used for drinking, there are additional risks of stomach and gastrointestinal cancer (Thabayneh 2015; Naskar et al. 2018; Khan 2021). According to a report published by the United States Environmental Protection Agency (USEPA), Rn-222 is the second major cause of lung cancer deaths every year, after smoking (EPA U 1991; EPA 1999; Sharma et al. 2017). Nayak et al. (2022) published a review work on groundwater radon distribution and associated health issues and its probable mitigation strategy. Çam-Kaynar and Parlak (2022) published their work on indoor radon measurement and the annual effective dose rates for different season dwellings in Manisa, Turkey. Therefore, assessment of radon in groundwater is absolutely essential for safe consumption and daily household usage, in addition to the usual estimation of Water Quality Index (WQI) for suitability for drinking purpose (Matta et al. 2020; Mohamed Amine et al. 2021).
Apart from all these conventional characteristics, due to high abundance of Rn-222 in groundwater, it is easy to evaluate the quantitative and qualitative groundwater study, knowing its path through the soil and rock formations. Due to the dissolution of several minerals, compounds and radioactive substances, Rn-222 activity in groundwater is much high on magnitude compared to the sea water (Nalukudiparambil et al. 2021; Singh et al. 2021). Therefore, the presence of Rn-222 in groundwater or soil can be used in the estimation of groundwater flow velocities, groundwater discharge rate, aquifer water residence time, detection of faults and fractures in the earth crust and as a tracer for earthquakes (Bertin and Bourg 1994; Hoehn and Von Gunten 1989; Naskar et al. 2022).
From research that has already been carried out all over the world, it is established that the concentration of radon in soil and groundwater undergoes wide variations due to various meteorological factors such as climatic conditions, soil permeability, soil porosity and soil temperature and due to geological features of a region such as active faults, fractures in the earth crust and types of rock present in that area (Ramola et al. 1998; Naskar et al. 2018; Shilpa et al. 2017; Arvela 1995; Kullab et al. 2001; Al-Khateeb et al. 2017; Rani et al. 2021). As the meteorological parameters change with seasons, it is necessary to conduct repeated measurements in all season to determine the seasonal variations of radon (Miklyaev and Petrova 2021; Rengan et al. 2022). Recent results of radon measurement across the world are enlisted in Table 1.
In a previous research work by the present group, four groundwater samples in the Purulia municipal area of Purulia district, West Bengal, were reported to have significantly high radon concentration (Mitra et al. 2021). Now, in order to study the possible reason behind its occurrence and associated health effects due to the consumption of this radon-rich water, more samples were analysed from this municipal area. Additionally, dissolved Na+, K+, Ca2+ and Li+ ions and the pH levels of these groundwater samples have been analysed with an aim to study their possible correlations with radon present in the samples.
Geological description of the study area
The present work focuses on the main urban centre of Purulia district, i.e. Purulia Municipality comprising 23 wards covering a total area around 14 square km (from 22°42′35″ N to 23°42″ N and from 85°49′ 25″ E to 86°57′ 37″E). According to the 2011 census, the municipality has a total population of 121,436 with a population density of about 6760 people per square km (District Census Handbook 2011; Chamling 2013). However, the population density as well as the population has increased in the last 10 years. A large portion of this population use tube-well water for domestic purposes. Geographically, the area is dominated by subtropical climate of high evaporation and low precipitation with a wide variation of temperature, minimum being 3.8 °C in winter while maximum being 52 °C in summer (Chamling 2013).
From the geological point of view, the study area lies in the Chotanagpur Granite Gneissic Complex (CGGC) of the eastern peninsular shield of India, which is well identified for mineral deposition (Sanyal and Sengupta 2012; Dolui et al. 2016; Mitra et al. 2021). The CGGC contains various stages of migmatites, granitoids, gneisses, amphibolites and granulites in different regions (Acharya and Nag 2013). Among them, exposed granites, Archaean-age gneisses and meta-sediments of pre-Cambrian age compose the bedrocks of the region (Sanyal and Sengupta 2012; Dolui et al. 2016). The composite rocks and soils and presence of a shear zone, namely, the South Purulia Shear Zone nearby, may raise the groundwater radon activity in this area (Otton 1992).
Sampling
A total of 120 groundwater samples were collected from deep tube-wells of depth ranging from about 20 m to about 60 m, from the 23 wards of Purulia municipality in such a way that there were at least two samples from each ward. Large population density and high daily usage of tube-well water for domestic purposes were considered for the selection of tube-wells in the municipal area. In order to ensure that the groundwater came from the desired depth, the tube-wells were first pumped for at least 5 − 10 min (Naskar et al. 2018; Mitra et al. 2021). Then, the mouths of the tube-wells were sealed using a football bladder and then pumped until the water rose up to the top of the tube-well, and it was kept for at least 30 min. Next, using 200-ml syringe water samples were collected from it and transferred into a 200-ml air-tight glass bottle to minimise radon loss during transportation. The glass bottles were then properly sealed and labelled. Additionally, water samples were taken into another 250 ml plastic bottle for measurement of dissolved Na+, K+, Ca2+, Li+ and properly sealed and labelled. Then, all the samples were brought into the laboratory for measurement. Water radon content of the samples were measured on the same day as the collection (delay time < 4 h). A global positioning system (GPS) meter was used to determine the sampling location.
Experimental methods
The temperatures and pHs of the water samples were measured in situ using a thermometer and a digital pH meter (HM_PH80) respectively. For the assessment of radon content in ground water samples, an online radon monitoring system composed of the ionisation chamber AlphaGuard DF2000 PRO and its accessory AquaKIT was used. This device is capable of measuring radon concentration in water from 0.01 Bq/l to 2 × 106 Bq/l with a maximum instrumental error of ± 0.3% (AlphaGuard Manual 2020; AquaKIT Manual 2017).
The following equation prescribed by the manufacturer (Naskar et al. 2018; Chowdhury et al. 2019) has been used to calculate radon concentration in water samples:
where Cwater is the radon concentration in water sample (Bq/l), Cair is radon concentration (Bq/m3) in the measuring set-up, C0 is the radon concentration in the measuring set-up before sample injection (Bq/m3), Vsystem is interior volume of the measurement setup (ml), Vsample is volume of the water sample (ml) and k is the radon diffusion coefficient. Diffusion coefficient value can be determined by using the following equation:
where T is the temperature of the water (°C)
The measurements were performed at room temperature so that the value of the diffusion coefficient was same as that provided by the manufacturer, and no more corrections were necessary.
The concentrations of dissolved Na+, K+, Ca2+ and Li+ ions in the water samples were measured using a micro controller-based Flame Photometer (G-301 Series, HPG Instruments, India) following the standard calibration procedure prescribed by the manufacturer. Firstly, the instrument was calibrated with two known activity solutions of the cations (Na+, K+, Ca2+, Li+). Calibration was done by using 10 mg/l and 50 mg/l standard solutions of each cation. Then, the instrument was set for the measurement mode for particular cations (Na+, K+, Ca2+, Li+) and 50 ml water sample was taken in a glass beaker and aspirated through a compressor to the flame. The flame colour changes according to the cation present in the water sample, and the attached micro-controller detector detects the activity in comparison to the standard solution, and the result is displayed. After aspirating with distilled water, the flame resets. This process was repeated four times, and the average of the data was considered.
Estimation of annual effective dose by inhalation and ingestion
The Rn-222 content in drinking water is comparatively more harmful than most other elements or ions (Cothern 2014). Radon can enter the human body in two ways: firstly, from consuming radon-containing water, i.e. ingestion, and secondly, from the release of dissolved radon from water and its inhalation and cause serious health issues (Binesh et al. 2010; Duggal et al. 2020). Therefore, to assess the risks regarding health issues in humans, it is necessary to evaluate the annual effective doses due to the inhalation and ingestion of radon. Doses associated with radon for adults, children and infants are calculated with the help of following equations.
The annual effective dose due to inhalation has been estimated by the following:
where RaW is the coefficient of transfer of radon from water to air (10−4), CRnW is the radon concentration in water (Bq/l), I is the average annual indoor occupancy time per individual (7000 h/y), F is the equilibrium factor between radon and its progeny (0.4) and DCF is the dose conversion factor for radon exposure (9 nSv/Bq/h/m3) (UNSCEAR 2000; UNSCER 2008; Naskar et al. 2018; Duggal et al. 2020).
The annual effective dose due to ingestion has been estimated using the following equation:
where CRnW is the radon concentration in water (Bq/l), CW is the average annual water intake (l/y) and EDC is the effective dose coefficient for ingestion (3.5 nSv/Bq) (UNSCEAR 2000; UNSCER 2008; Mittal et al. 2016).
Result and discussion
The pH and the concentrations of radon and some major cations in 120 groundwater samples have been estimated in Purulia municipality of Purulia district, West Bengal, India. The measured values of pH of the water samples were found to range from 6.4 to 9.0 with an average of 7.70, indicating that the samples were mildly acidic to alkaline in nature. It has been observed that these ground water samples contain widely varying concentration of radon ranging from 10.44 to 403.56 Bq/l with an average of 108.70 Bq/l. The sampling locations along with the distribution of radon concentrations have been depicted in the bubble plot of Fig. 1. The concentration profiles of radon as well as cations (Na+, K+, Ca2+, Li+) and pH have been portrayed in the bar plot of Fig. 2. The wide variety of radon values, as reflected in these numbers and bar plots can be inferred to be a result of distribution of rocks containing different kinds of minerals, nature of the bedrocks, the presence of geological faults and fractures in the study area, as well as the depths of the tube-wells. From Fig. 1, it can be observed that the apparently high values of radon only appear in the southern part of Purulia Municipality where the tube-wells are deep. This may also show some indication of an unknown geological fault line which can provide a pathway for radon from deep inside the earth’s crust to travel to the upper layers and dissolve into the aquifer waters (Chowdhury et al. 2019; Mitra et al. 2021). The sample density in this region with high radon activity has been increased to understand the proper distribution of radon as well as certain cations (Na+, K+, Ca2+, Li+) and pH. As discussed earlier, the bedrocks of this region are mainly composed of granitic rocks, which are capable of trapping radon in their pores. This trapped radon may also dissolve into groundwater and may be another reason for high radon occurrence. On the other hand, the low concentrations of groundwater radon in certain locations may be due to water coming from less depth.
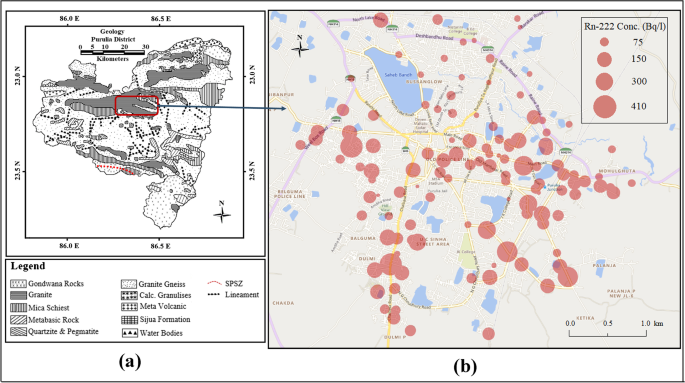
(Source: Geological Survey of India and modified after Sanyal and Sengupta 2012)
Bubble plot of the measured radon concentration in water samples with locations of the study area
In order to compare the water radon activity data obtained in this study with earlier studies carried out in different parts of the world, radon activity data from some of those studied are presented in Table 1. These data give an idea about how groundwater radon concentration changes from one place to another depending upon geological and meteorological parameters. The data in Table 1 also show that the average radon concentration in the study area is much higher than that in many parts of the world.
As for the cations, the concentration of Ca2+ ion was found to vary from 24.4 to 691.0 mg/l with an average of 164.7 mg/l, with 77% of the samples exceeding the maximum permissible limit of Ca2+ in drinking water (75 mg/l) prescribed by WHO (Kumar and Puri 2012). The high abundance of Ca2+ may be due to the presence of gypsum and carbonate in the underlying sedimentary bed rocks (Telahigue et al. 2018). Moreover, cation exchange reactions can raise the concentration of Ca2+, while water flows upward from deep aquifer (Maoui et al. 2010).
Na+ was found to vary between 12.8 and 452.0 mg/l in the groundwater samples, with an average of 87.7 mg/l. In this case, all the samples exceed the maximum permissible limit of 20 mg/l (WHO 1996; NYS:DoH 2019) except two samples (W-69, W-55). The major source of Na+ in the water samples can be the cation exchange reactions, water–rock interaction and mixing of saline water deep down the aquifer (Daniele et al. 2013; Telahigue et al. 2018).
The samples were not found to contain significant amounts of K+ and Li+. The concentrations of K+ dissolved in the water samples were found to lie between 0.3 and 100.0 mg/l with an average of 5.8 mg/l. Thirteen percent of the samples contain K+ above the prescribed consumption limit of 8 mg/l (WHO 1996). The concentrations of Li+ in 19 samples were found to be below detection limit (BDL), and in the rest of the samples the maximum was 0.70 mg/l.
For the statistical analysis of the dataset, initially all the values have been taken into consideration. Statistical parameters such as mean, median, standard deviation and correlations between the parameters have been estimated and listed in Table 2. It has been observed that when all the data was included, the Pearson correlation coefficient between radon and Na+, K+, Li+, Ca2+ and pH are − 0.18, − 0.11, − 0.04, − 0.21 and 0.06, respectively, as depicted in Fig. 3. It can be concluded from Fig. 3 that there is almost no correlation between radon and Li+ and pH, while slight negative correlations between radon and Na+, K+ and Ca2+ were seen. In order to find the variation of all the measured cations (Na+, K+, Ca2+, Li+) and pH with each other, linear fit curves have been plotted and shown in Fig. 4. From the Pearson correlation coefficients for all the plots of Fig. 4, it is observed that there is moderately positive correlation between Na+ and Ca2+, Na+ and Li+ and pH and Li+, whereas slightly negative correlation has been found between pH and Ca2+.
Since the sample size is 120, and some of the samples have radon and/or cation concentrations much higher than the average, statistical analysis is distorted. Therefore, it is necessary to discard the comparatively higher values for a detailed statistical analysis using a standard statistical method of elimination. The values higher than the limit of mean plus twice the standard deviation (M + 2σ) have been discarded, and the descriptive statistical analysis is performed and tabulated in Table 3. It should be noted that a positive value of skewness indicates that the distribution has a long tail while a negative value of skewness implies a tail at the beginning of the distribution (Press et al. 2007). Another important statistical parameter is kurtosis, which describes the peakedness of the distribution. From the value of the parameter excess kurtosis (kurtosis – 3), an idea about the distribution can be obtained. If the value of the excess kurtosis is exactly zero, the distribution is called normal distribution (Kim 2013). In this study, the value of the excess kurtosis is negative for radon, Na+, Ca2+ and pH, which implies that the distribution is flat-topped curve and possesses broad distribution. On the other hand, value of the excess kurtosis is positive for K+ which corresponds to high peak and a narrow distribution (Sharma and Ojha 2020). The Pearson’s correlation coefficients between radon and Na+, K+, Ca2+, Li+ and pH are − 0.16, − 0.19, − 0.18, 0.03 and 0.16 respectively, and are depicted in Fig. 5. Thus, low negative correlation has been found between radon and all the cations except Li+ and pH. Similar correlation coefficients have been estimated for the cations and pH also. These coefficients obtained for different combination are depicted in Fig. 6. From the Pearson’s correlation coefficient of the linear fit curve (Fig. 6), it is observed that there is a positive correlation between Ca2+ and Na+ and K+, and moderately negative correlation is found between Ca2+ and pH. Remaining correlation coefficients show no significant relation except a low positive correlation between Li+ and K+.
Dissolved radon in drinking water is the principal source of radiation doses to the population. The doses due to inhalation and ingestion have been evaluated using Eqs. (3) and (4), and these two have been added to get the total Annual Effective Dose (AED). These values are portrayed in Fig. 7 for different age groups, i.e. adults, children and infants. For the calculation of the ingestion dose associated with radon, the amount of water consumption is of great importance. Since the area of study lie in a substantially dry and humid region, an adult usually consumes ~ 4.5 L of water per day. The average water intake for children and infants are considered 2 and 0.75 L per day respectively (Ademola and Oyeleke 2017; Mitra et al. 2021). The total AED for adults, children and infants has been found to vary in the range of 0.06 to 2.42 mSv/y, 0.03 to 1.13 mSv/y and 0.01 to 0.49 mSv/y respectively, with an average of 0.65 mSv/y, 0.30 mSv/y and 0.13 mSv/y respectively. The RDL for annual effective dose recommended by European Union Commission and WHO is 0.1 mSv/y (Water, Sanitation and Health Team and World Health Organization 2004 and EU 2005). Now, from Fig. 7, it can be seen that, though there is a wide variation among the doses in the study area, 21% of the samples exceed the RDL of 0.1 mSv/y, and are even at ~ 10 − 20 times higher than the RDL. This may be due to the fact that the area from where the samples were collected could be granite-rich, and the tube-wells may also be deeper, which lead to the high radon concentration in groundwater and the corresponding high doses.
Conclusion
The concentration of radon in 34% of the groundwater samples under study has been found to exceed the value of 100 Bq/l for which controls to be implemented as per guidelines prescribed by World Health Organization and European Union Commission (Water, Sanitation and Health Team and World Health Organization 2004; EU commission 2001), while all samples surpass the limit of 11.1 Bq/l radon concentration in drinking water set by USEPA (United States Environmental Protection Agency) 1999. In addition, it has been observed that the total annual effective doses due to water radon in adults (≥ 17 years), children (2 − 17 years) and infants (≤ 1 year) are significantly higher than the RDL of 0.1 mSv/y prescribed by WHO and European Council. To mitigate some of the health consequences, the local people may be suggested to boil the water before drinking, as boiling expels dissolved radon from water.
Regarding the presence of cations in groundwater, correlations between radon and pH and some cations (Na+, K+, Ca2+) have been found to be weakly negative. This work would provide a baseline data for further study of water radon in the region, radon mapping of the study area, and may also be helpful in determining the Water Quality index (WQI). However, it is necessary to perform this type work repeatedly in all seasons in order to estimate the seasonal variation and the exact health risk to the inhabitants of this study area.
Data availability
The datasets used and/or analysed in the current study would be available from the corresponding author on reasonable request.
References
Abdallah SM, Habib RR, Nuwayhid RY, Chatila M, Katul G (2007) Radon measurements in well and spring water in Lebanon. Radiat Meas 42(2):298–303. https://doi.org/10.1016/j.radmeas.2006.11.004
Abojassim AA (2020) Comparative study between active and passive techniques for measuring radon concentrations in groundwater of Al-Najaf city, Iraq. Groundw Sustain Dev 11:100476. https://doi.org/10.1016/j.gsd.2020.100476
Acharya T, Nag SK (2013) Study of groundwater prospects of the crystalline rocks in Purulia District, West Bengal, India using remote sensing data. Earth Resour 1(2):54–59. https://doi.org/10.12966/er.07.03.2013
Ademola JA, Oyeleke OA (2017) Radon-222 in groundwater and effective dose due to ingestion and inhalation in the city of Ibadan, Nigeria. J Radiol Prot 37(1):189. https://doi.org/10.1088/1361-6498/37/1/189
Agoubi B (2021) Review: origin, heating process, and groundwater flow system of non-volcanic thermal aquifers in Tunisia. Arab J Geosci 14:369. https://doi.org/10.1007/s12517-021-06632-3
Al-Khateeb HM, Nuseirat M, Aljarrah K, Al-Akhras MAH, Bani-Salameh H (2017) Seasonal variation of indoor radon concentration in a desert climate. Appl Radiat Isot 130:49–53. https://doi.org/10.1016/j.apradiso.2017.08.017
AlphaGuard Manual (2020) https://drive.google.com/file/d/1ZzKVpL1PWEuFlg2m7dHfWXFCtMG4fHM0/view?usp=sharing
AquaKIT Manual (2017) https://www.bertin-instruments.com/wp-content/uploads/secured-file/AquaKIT-Brief-Instruction_E.pdf
Arvela H (1995) Seasonal variation in radon concentration of 3000 dwellings with model comparisons. Radiat Prot Dosimetry 59(1):33–42. https://doi.org/10.1093/oxfordjournals.rpd.a082634
Aydin MF, Söǧüt Ö (2019) Measurement of radon gas activity concentrations in drinking water in the city center of Adıyaman, Turkey. Radiat Prot Environ 42(1):10. https://doi.org/10.4103/rpe.RPE_54_18
Bem H, Plota U, Staniszewska M, Bem EM, Mazurek D (2014) Radon (222Rn) in underground drinking water supplies of the Southern Greater Poland Region. J Radioanal Nucl Chem 299(3):1307–1312. https://doi.org/10.1007/s10967-013-2912-1
Bertin C, Bourg AC (1994) Radon-222 and chloride as natural tracers ofthe infiltration of river water into an alluvial aquifer in which there is significant river/groundwater mixing. Environ Sci Technol 28(5):794–798. https://doi.org/10.1021/es00054a008
Binesh A, Mohammadi S, Mowlavi AA, Parvaresh P (2010) Evaluation of the radiation dose from radon ingestion and inhalation in drinking water. Int J Water Resour Environ Eng 2(7):174–178. https://doi.org/10.5897/IJWREE.9000019
Branco R, Cruz JV, Silva C, Coutinho R, Andrade C, Zanon V (2021) Radon (222Rn) occurrence in groundwater bodies on São Miguel Island (Azores archipelago, Portugal). Environ Earth Sci 80(17):1–14. https://doi.org/10.1007/s12665-021-09906-x
Çam-Kaynar S, Parlak Y (2022) Indoor radon concentrations and annual effective dose rates for spring and summer seasons by using CR-39 nuclear track detectors in dwellings in Manisa, Turkey. Arab J Geosci 15(17):1–8
Chamling M (2013) Critical appraisal of urbanization in Purulia municipality. Geo-Analyst 50–58
Cho BW, Kim HK, Kim MS, Hwang JH, Yoon U, Cho SY, Choo CO (2019) Radon concentrations in the community groundwater system of South Korea. Environ Monit Assess 191(3):1–10. https://doi.org/10.1007/s10661-019-7301-y
Chowdhury S, Barman C, Deb A, Raha S, Ghose D (2019) Study of variation of soil radon exhalation rate with meteorological parameters in Bakreswar-Tantloi geothermal region of West Bengal and Jharkhand, India. J Radioanal Nucl Chem 319(1):23–32. https://doi.org/10.1007/s10967-018-6286-2
Cothern CR (2014) Radon, radium, and uranium in drinking water. CRC Press, Boca Raton
Cucu MI, Dupleac D (2021) The contribution of the radioactive gas, radon, to the effective dose received by the population of Mioveni City, Arges County, Romania. Educ Res (IJMCER) 3(4):131–139
Daniele L, Vallejos Á, Corbella M, Molina L, Pulido-Bosch A (2013) Hydrogeochemistry and geochemical simulations to assess water–rock interactions in complex carbonate aquifers: the case of Aguadulce (SE Spain). Appl Geochem 29:43–54. https://doi.org/10.1016/j.apgeochem.2012.11.011
Deeba F, Rahman SH, Kabir MZ (2020) Radon Concentration in soil and groundwater of west coastal area, Bangladesh. Radiat Prot Dosim 191(3):341–348. https://doi.org/10.1093/rpd/ncaa134
District Census Handbook (2011) https://censusindia.gov.in/nada/index.php/catalog/1354
Dolui G, Chatterjee S, Chatterjee ND (2016) Geophysical and geochemical alteration of rocks in granitic profiles during intense weathering in southern Purulia district, West Bengal, India. Model Earth Syst Environ 2(3):132. https://doi.org/10.1007/s40808-016-0188-5
Duggal V, Sharma S, Mehra R (2020) Risk assessment of radon in drinking water in Khetri Copper Belt of Rajasthan, India. Chemosphere 239:124782. https://doi.org/10.1016/j.chemosphere.2019.124782
EPA U (1991) Environmental Protection Agency, National primary drinking water regulations: radio nuclides; proposed rules. Fed Reg 56(138):33050
EPA U (1999) Radon in drinking water health risk reduction and cost analysis. Fed Reg 64(38):9560–9599
EU (2005) European Union Commission. Progress Report. Brussels, 9 November 2005. SEC (2005) 1426
EU (2001) European Union Commission Recommendation on the protection of the public against exposure to radon in drinking water supplies. Off J Eur Commun 344:85–88
Günay O, Aközcan S, Kulal F (2019) Measurement of indoor radon concentration and annual effective dose estimation for a university campus in Istabul. Arab J Geosci 12(5):1–8. https://doi.org/10.1007/s12517-019-4344-x
Hoehn E, Von Gunten HR (1989) Radon in groundwater: a tool to assess infiltration from surface waters to aquifers. Water Resour Res 25(8):1795–1803. https://doi.org/10.1029/WR025i008p01795
Isinkaye MO, Matthew-Ojelabi F, Adegun CO, Fasanmi PO, Adeleye FA, Olowomofe OG (2021) Annual effective dose from 222Rn in groundwater of a Nigeria University campus area. Appl Water Sci 11(5):1–10. https://doi.org/10.1007/s13201-021-01417-1
Jaishi HP, Singh S, Tiwari RP, Tiwari RC (2014) Analysis of soil radon data in earthquake precursory studies. Ann Geophys 57(5):S0544–S0544. https://doi.org/10.4401/ag-6513
Kareem DO, Ibrahim AA, Ibrahiem OS (2020) Heavy metal and radon gas concentration levels in Khasa River in Kirkuk City (NE Iraq) and the associated health effects. Arab J Geosci 13(19):1–11. https://doi.org/10.1007/s12517-020-06037-8
Kessongo J, Bahu Y, Inácio M, Peralta L, Soares S (2020) Radon concentration potential in Bibala municipality water: consequences for public consumption. Radiat Phys Chem 173:108951. https://doi.org/10.1016/j.radphyschem.2020.108951
Khan MS (2021) Measurements of lung doses from radon and thoron in the dwellings of Al-Zulfi, Saudi Arabia, for the assessment of health risk due to ionizing radiation. Arab J Geosci 14(12):1–10. https://doi.org/10.1007/s12517-021-07448-x
Kim HY (2013) Statistical notes for clinical researchers: assessing normal distribution (2) using skewness and kurtosis. Restor Dent Endod 38(1):52–54. https://doi.org/10.5395/rde.2013.38.1.52
Kullab MK, Al-Bataina BA, Ismail AM, Abumurad KM (2001) Seasonal variation of radon-222 concentrations in specific locations in Jordan. Radiat Meas 34(1–6):361–364. https://doi.org/10.1016/S1350-4487(01)00186-X
Kumar M, Puri A (2012) A review of permissible limits of drinking water. Indian J Occup Environ Med 16(1):40. https://doi.org/10.4103/0019-5278.99696
Lima-Flores A, Castaño VM, Golzarri JI, Chavarría-Sánchez AC, Espinosa G (2021) Radon in drinking water in Mexico City. J Radioanal Nucl Chem 329(2):527–536. https://doi.org/10.1007/s10967-021-07849-y
Maoui A, Kherici N, Derradji F (2010) Hydrochemistry of an Albian sandstone aquifer in a semi arid region, Ain oussera, Algeria. Environ Earth Sci 60(4):689–701. https://doi.org/10.1007/s12665-009-0207-1
Matta G, Kumar A, Nayak A, Kumar P, Kumar A, Tiwari AK (2020) Determination of water quality of Ganga River System in Himalayan region, referencing indexing techniques. Arab J Geosci 13(19):1–11
Miklyaev PS, Petrova TB (2021) Study of abnormal seasonal variations in the radon exhalation rate in a fault zone. Geochem Int 59(4):435–447
Mitra S, Chowdhury S, Mukherjee J, Sutradhar S, Mondal S, Barman C, Deb A (2021) Assessment of radon (222Rn) activity in groundwater and soil-gas in Purulia district, West Bengal, India. J Radioanal Nucl Chem 330:1331–1338. https://doi.org/10.1007/s10967-021-07989-1
Mittal S, Rani A, Mehra R (2016) Estimation of radon concentration in soil and groundwater samples of Northern Rajasthan, India. J Radiat Res Appl Sci 9(2):125–130. https://doi.org/10.1016/j.jrras.2015.10.006
Mohamed Amine B, Zeghadnia L, Nesrine B, Matta G, Boranen S (2021) Drinking water quality assessment using principal component analysis: case study of the town of Souk Ahras, Algeria. Egypt J Chem 64(6):3069–3075
Nalukudiparambil J, Gopinath G, Ramakrishnan RT, Surendran AK (2021) Groundwater radon (222Rn) assessment of a coastal city in the high background radiation area (HBRA), India. Arab J Geosci 14(8):1–7. https://doi.org/10.1007/s12517-021-07082-7
Naskar AK, Gazi M, Barman C, Chowdhury S, Mondal M, Ghosh D, Deb A (2018) Estimation of underground water radon danger in Bakreswar and Tantloi Geothermal Region, India. J Radioanal Nucl Chem 315(2):273–283. https://doi.org/10.1007/s10967-017-5668-1
Naskar AK, Gazi M, Mondal M, Deb A (2022) Water radon risk in Susunia hill area: an assessment in terms of radiation dose. Environ Sci Pollut Res 29(8):11160–11171
National Research Council (1999) Risk assessment of radon in drinking water. The National Academies Press, Washington, DC. https://doi.org/10.17226/6287
Nayak T, Basak S, Deb A, Dhal PK (2022) A systematic review on groundwater radon distribution with human health consequences and probable mitigation strategy. J Environ Radioact 247:106852
Nuhu H, Hashim S, Sanusi MSM, Saleh MA (2020) Radon activity concentration measurements in water sources from Perak state Malaysia. J Radiat Res Appl Sci 13(1):665–671. https://doi.org/10.1080/16878507.2020.1820270
NYS:DoH (2019) https://www.health.ny.gov/environmental/water/drinking/salt_drinkingwater.htm
Otton JK (1992) The geology of radon. Government Printing Office, Washington
Press WH, Teukolsky SA, Vetterling WT, Flannery BP (2007) Numerical recipes: the art of scientific computing, 3rdedn, Cambridge University Press
Qadir RW, Asaad N, Qadir KW, Ahmad ST (2021) Relationship between radon concentration and physicochemical parameters in groundwater of Erbil city, Iraq. J Radiat Res Appl Sci 14(1):61–69. https://doi.org/10.1080/16878507.2020.1856588
Rahimi M, Abadi AAM, Koopaei LJ (2022) Radon concentration in groundwater, its relation with geological structure and some physicochemical parameters of Zarand in Iran. Appl Radiat Isot 185:110223. https://doi.org/10.1016/j.apradiso.2022.110223
Ramola RC, Kandari MS, Rawat RBS, Ramachandran TV, Choubey VM (1998) A study of seasonal variations of radon levels in different types of houses. J Environ Radioact 39(1):1–7. https://doi.org/10.1016/S0265-931X(97)00049-0
Rangaswamy DR, Srinivasa E, Srilatha MC, Sannappa J (2016) Measurement of radon concentration in drinking water of Shimoga district, Karnataka, India. J Radioanal Nucl Chem 307(2):907–916. https://doi.org/10.1007/s10967-015-4216-0
Rani S, Kansal S, Singla AK, Mehra R (2021) Radiological risk assessment to the public due to the presence of radon in water of Barnala district, Punjab, India. Environ Geochem Health 43(12):5011–5024
Rengan AG, Joseph S, Sellamuthu S (2022) Seasonal and geological controls of radon (222Rn) in groundwater of Vamanapuram river basin, SW India. Geocarto Int 1–26. https://doi.org/10.1080/10106049.2022.2142961
Romano D, Magazù S, Sabatino G, Di Bella M, Tripodo A, Nania G, ..., Italiano F (2022) Radon concentration in groundwater of north-eastern Sicily (Italy). J Inst 17(09): P09003
Rotich CK, Hashim NO, Chege MW, Nyambura C (2020) Measurement of radon activity concentration in underground water of Bureti sub-county of Kericho county Kenya. Radiat Prot Dosimetry 192(1):56–60. https://doi.org/10.1093/rpd/ncaa193
Sanyal S, Sengupta P (2012) Metamorphic evolution of the Chotanagpur granite gneiss complex of the east Indian shield: current status. Geol Soc Lond Spec Publ 365(1):117–145. https://doi.org/10.1144/SP365.7
Sharma S, Duggal V, Srivastava AK, Mehra R (2017) Assessment of radiation dose from exposure to radon in drinking water from Western Haryana, India. Int J Environ Res 11(2):141–147. https://doi.org/10.1007/s41742-017-0015-5
Sharma C, Ojha CSP (2020) Statistical parameters of hydrometeorological variables: standard deviation, SNR, skewness and kurtosis. In: AlKhaddar R, Singh R, Dutta S, Kumari M. (eds) Advances in Water Resources Engineering and Management. Lecture Notes in Civil Engineering, vol 39. Springer, Singapore. https://doi.org/10.1007/978-981-13-8181-2_5
Shilpa GM, Anandaram BN, Mohankumari TL (2017) Measurement of 222Rn concentration in drinking water in the environs of Thirthahalli taluk, Karnataka, India. J Radiat Res Appl Sci 10(3):262–268. https://doi.org/10.1016/j.jrras.2017.05.007
Singh J, Singh H, Singh S, Bajwa BS (2008) Estimation of uranium and radon concentration in some drinking water samples. Radiat Meas 43:S523–S526. https://doi.org/10.1016/j.radmeas.2008.04.004
Singh P, Nautiyal OP, Joshi M, Kumar A, Ahamad T, Singh K (2021) Assessment of physicochemical and radon-attributable radiological parameters of drinking water samples of Pithoragarh district, Uttarakhand. J Radioanal Nucl Chem 330(3):1559–1570
Tan W, Li Y, Tan K, Xie Y, Han S, Wang P (2019) Distribution of radon and risk assessment of its radiation dose in groundwater drinking for village people nearby the W-polymetallic metallogenic district at Dongpo in southern Hunan province, China. Appl Radiat Isot 151:39–45. https://doi.org/10.1016/j.apradiso.2019.05.008
Telahigue F, Agoubi B, Souid F, Kharroubi A (2018) Groundwater chemistry and radon-222 distribution in Jerba Island, Tunisia. J Environ Radioact 182:74–84. https://doi.org/10.1016/j.jenvrad.2017.11.025
Thabayneh KM (2015) Measurement of 222Rn concentration levels in drinking water and the associated health effects in the Southern part of West bank–Palestine. Appl Radiat Isot 103:48–53. https://doi.org/10.1016/j.apradiso.2015.05.007
UNSCEAR (2000) Sources and effects of atomic radiation, report to General Assembly, Annex B. United Nations, New York
UNSCER (2008) United Nations. Scientific committee on the effects of atomic radiation. Report of the United Nations Scientific Committee on the Effects of Atomic Radiation: Fifty-sixth Session (10–18 July 2008) (No. 46). United Nations Publications
USEPA (United States Environmental Protection Agency) (1999) Proposed radon in drinking water rule technical fact sheet. https://nepis.epa.gov/Exe/ZyPURL.cgi?Dockey=P1008HLV.txt
Water: Consequences for public consumption. Radiat Phys Chem 173:108951
Water, Sanitation and Health Team and World Health Organization (2004) Guidelines for drinking-water quality, vol 1, recommendations, 3rd edn. World Health Organization. https://apps.who.int/iris/handle/10665/42852
World Health Organization & International Programme on Chemical Safety (1996) Guidelines for drinking-water quality. Vol. 2, Health criteria and other supporting information, 2nd ed. World Health Organization. https://apps.who.int/iris/handle/10665/38551
Acknowledgements
We are grateful to Sidho-Kanho-Birsha University for providing the essential infrastructural facility and also the local people of the area under study for their cooperation.
Funding
CB acknowledges the financial assistance from Department of Science & Technology-Fund for Improvement of Science &Technology Infrastructure (SR/FST/PS-1/2020/159) New Delhi, India; RUSA grant of SKBU; University Grant Commissions-Faculty Research Promotion Scheme Start-up-Grant [(No.F.30–557/2021(BSR) Dated: 21 Jan, 2022)], seed grant of faculty research, SKBU.
Author information
Authors and Affiliations
Contributions
Joydeep Mukherjee, Sayantan Mitra, Sushanta Sutradhar and Saheli Chowdhury analysed and interpreted the water radon data regarding this work. Joydeep Mukherjee, Sushanta Sutradhar and Sayantan Mitra collected the water samples and measured the radon activity using the AlphaGuard radon monitor and pH of the water samples using digital pH meter. Joydeep Mukherjee and Sayantan Mitra were major contributors in writing the manuscript. The overall corrections were made by Saheli Chowdhury, Sonjoy Mondal, Argha Deb and Chiranjib Barman. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Amjad Kallel
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mukherjee, J., Mitra, S., Sutradhar, S. et al. Analysis of radon concentration in ground water and estimation of associated health risks in Purulia Municipality, West Bengal, India. Arab J Geosci 16, 125 (2023). https://doi.org/10.1007/s12517-023-11202-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12517-023-11202-w










