Abstract
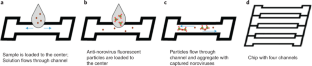
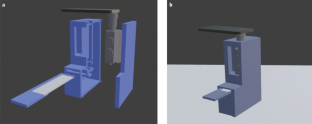
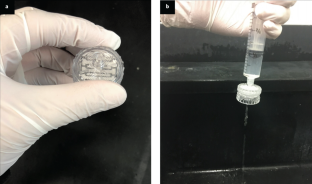
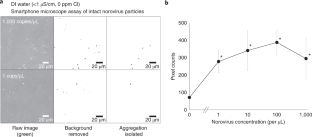
Norovirus is a widespread public health threat and has a very low infectious dose. This protocol presents the extremely sensitive mobile detection of norovirus from water samples using a custom-built smartphone-based fluorescence microscope and a paper microfluidic chip. Antibody-conjugated fluorescent particles are immunoagglutinated and spread over the paper microfluidic chip by capillary action for individual counting using a smartphone-based fluorescence microscope. Smartphone images are analyzed using intensity- and size-based thresholding for the elimination of background noise and autofluorescence as well as for the isolation of immunoagglutinated particles. The resulting pixel counts of particles are correlated with the norovirus concentration of the tested sample. This protocol provides detailed guidelines for the construction and optimization of the smartphone- and paper-based assay. In addition, a 3D-printed enclosure is presented to incorporate all components in a dark environment. On-chip concentration and the assay of higher concentrations are presented to further broaden the assay range. This method is the first to be presented as a highly sensitive mobile platform for norovirus detection using low-cost materials. With all materials and reagents prepared, a single standard assay takes under 20 min. Although the method described is used for detection of norovirus, the same protocol could be adapted for detection of other pathogens by using different antibodies.
This is a preview of subscription content, access via your institution
Access options
Access to this article via Institution of Civil Engineers Library is not available.
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
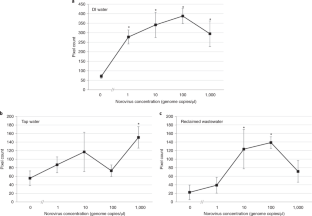
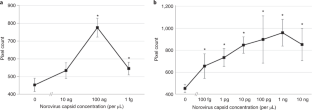
The data that support the findings of this study either were previously published in ref. 3 (Figs. 6 and 7, with source data provided in Supplementary Data 3) or are available upon reasonable request from the corresponding author (Figs. 8 and 9, with source data is provided in Supplementary Data 4 and 5). The video clip demonstrating the enclosure assembly is available in Supplementary Video 1. The video clip demonstrating the assay procedure is available in Supplementary Video 2.
Code availability
The SolidWorks designs of paper microfluidic chip and microscope enclosure are available in Supplementary Data 1. MATLAB Mobile, MATLAB, and ImageJ macro codes are available in Supplementary Data 2, along with raw sample images. These codes are also deposited and publicly available at https://github.com/jeongyeolyoon/yoon-lab. The code in this protocol has been peer-reviewed.
References
Teunis, P. F. M. et al. Norwalk virus: how infectious is it? J. Med. Virol. 80, 1468–1476 (2008).
Greening, G. E. & Cannon, J. L. Human and animal viruses in food (including taxonomy of enteric viruses). in Viruses in Foods (eds Goyal, S.M. & Cannon, J.L.) 5-57 (Springer, 2016). https://doi.org/10.1007/978-3-319-30723-7_2
Chung, S. et al. Smartphone-based paper microfluidic particulometry of norovirus from environmental water samples at single copy level. ACS Omega 4, 11180–11188 (2019).
Park, T. S. & Yoon, J.-Y. Smartphone detection of Escherichia coli from field water samples on paper microfluidics. IEEE Sens. J. 15, 1902–1907 (2015).
Park, T. S., Li, W., McCracken, K. E. & Yoon, J.-Y. Smartphone quantifies Salmonella from paper microfluidics. Lab Chip 13, 4832–4840 (2013).
Klug, K. E., Reynolds, K. A. & Yoon, J.-Y. A capillary flow dynamics-based sensing modality for direct environmental pathogen monitoring. Chem. Eur. J. 24, 6025–6029 (2018).
Ulep, T.-H. et al. Smartphone based on-chip fluorescence imaging and capillary flow velocity measurement for detecting ROR1+ cancer cells from buffy coat blood samples on dual-layer paper microfluidic chip. Biosens. Bioelectron. 153, 112042 (2020).
Kwon, H.-J., Dean, Z. S., Angus, S. V. & Yoon, J.-Y. Lab-on-a-chip for field Escherichia coli assays: long-term stability of reagents and automatic sampling system. JALA 15, 216–223 (2010).
Yoon, J.-Y. Introduction to Biosensors: From Electric Circuits to Immunosensors 2nd edn, 153–170 (Springer, 2016). https://doi.org/10.1007/978-3-319-27413-3
Ulep, T.-H. & Yoon, J.-Y. Challenges in paper-based fluorogenic optical sensing with smartphones. Nano Converg. 5, 14 (2018).
Ettayebi, K. et al. Replication of human noroviruses in stem cell–derived human enteroids. Science 353, 1387–1393 (2016).
Coudray-Meunier, C. et al. A comparative study of digital RT-PCR and RT-qPCR for quantification of Hepatitis A virus and Norovirus in lettuce and water samples. Int. J. Food Microbiol. 201, 17–26 (2015).
Rivadulla, E. & Romalde, J. L. A comprehensive review on human Aichi virus. Virol. Sin. https://doi.org/10.1007/s12250-020-00222-5 (2020).
de Bruin, E., Duizer, E., Vennema, H. & Koopmans, M. P. Diagnosis of Norovirus outbreaks by commercial ELISA or RT-PCR. J. Virol. Meth. 137, 259–264 (2006).
Park, J. P., Cropek, D. M. & Banta, S. High affinity peptides for the recognition of the heart disease biomarker troponin I identified using phage display. Biotechnol. Bioeng 105, 678–686 (2010).
Burton-Macleod, J. A. et al. Evaluation and comparison of two commercial enzyme-linked immunosorbent assay kits for detection of antigenically diverse human noroviruses in stool samples. J. Clin. Microbiol. 42, 2587–2595 (2004).
Dimitriadis, A., Bruggink, L. D. & Marshall, J. A. Evaluation of the Dako IDEIA norovirus EIA assay for detection of norovirus using faecal specimens from Australian gastroenteritis outbreaks. Pathology 38, 157–165 (2006).
Richards, A. F. et al. Evaluation of a commercial ELISA for detection Norwalk-like virus antigen in faeces. J. Clin. Virol. 26, 109–115 (2003).
Okitsu-Negishi, S. et al. Detection of Norovirus antigens from recombinant virus-like particles and stool samples by a commercial Norovirus enzyme-linked immunosorbent assay kit. J. Clin. Microbiol. 44, 3784–3786 (2006).
Mansfield, M. A. in Lateral Flow Assays (eds Wong, R. & Tse, H.) 1–19 (Humana Press, 2009).
Li, H., Han, D., Hegener, M. A., Pauletti, G. M. & Steckl, A. J. Flow reproducibility of whole blood and other bodily fluids in simplified no reaction lateral flow assay devices. Biomicrofluidics 11, 024116 (2017).
Cho, S., Park, T. S., Nahapetian, T. & Yoon, J.-Y. Smartphone-based, sensitive μPAD detection of urinary tract infection and gonorrhea. Biosens. Bioelectron. 74, 601–611 (2015).
Yoon, J.-Y., Park, H.-Y., Kim, J.-H. & Kim, W.-S. Adsorption of BSA on highly carboxylated microspheres—quantitative effects of surface functional groups and interaction forces. J. Colloid Interface Sci. 177, 613–620 (1996).
Cho, S., Park, T. S., Reynolds, K. A. & Yoon, J.-Y. Multi-normalization and interpolation protocol to improve norovirus immunoagglutination assay from paper microfluidics and smartphone detection. SLAS Technol. 23, 30–43 (2018).
Eyupoglu, C. Implementation of Bernsen’s locally adaptive binarization method for gray scale images. Online J. Sci. Technol. 7, 68–72 (2017).
Acknowledgements
The authors thank S. Perea for his contribution to an earlier version of this work. This work was funded by the University of Arizona National Science Foundation Water and Environmental Technology (WET) Center (award number IIP-1361815) and Tucson Water.
Author information
Authors and Affiliations
Contributions
J.-Y.Y. conceived the overall concept with input from S.C., L.E.B., W.Q.B., and K.A.R. S.C., L.E.B. and C.M.J. designed, fabricated, and tested the paper microfluidic chips, smartphone-based fluorescence microscope and its 3D-printed enclosure, with input from J.-Y.Y. Protocols for optimizing antibody conjugation to particles, paper types, and on-chip concentration were conceived and developed by S.C., L.E.B. and J.-Y.Y., with input from W.Q.B. and K.A.R. Image-processing algorithms and codes were conceived and developed by S.C., L.E.B., and A.G. with input from J.-Y.Y. Water and norovirus sample preparations and procedures were developed and tested by C.M.M. and W.Q.B. L.E.B. and J.-Y.Y. wrote the manuscript with input from S.C., W.Q.B. and K.A.R. All authors have given approval of the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Protocols thanks Suresh Neethirajan, Daniel Olson and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key references using this protocol
Chung, S. et al. ACS Omega 6, 11180−11188 (2019): https://doi.org/10.1021/acsomega.9b00772
Ulep, T.-H. et al. Biosens. Bioelectron. 153, 112042 (2020): https://doi.org/10.1016/j.bios.2020.112042
Key data used in this protocol
Chung, S. et al. ACS Omega 6, 11180−11188 (2019): https://doi.org/10.1021/acsomega.9b00772
Supplementary information
Supplementary Information
Supplementary Figs. 1–5.
Supplementary Data 1
The SolidWorks designs of paper microfluidic chip and microscope enclosure
Supplementary Data 2
MATLAB Mobile code, MATLAB code, and ImageJ macro code, along with raw image examples
Supplementary Video 1
Video clip demonstrating the enclosure assembly
Supplementary Video 2
Video clip demonstrating the assay procedure
Rights and permissions
About this article
Cite this article
Chung, S., Breshears, L.E., Gonzales, A. et al. Norovirus detection in water samples at the level of single virus copies per microliter using a smartphone-based fluorescence microscope. Nat Protoc 16, 1452–1475 (2021). https://doi.org/10.1038/s41596-020-00460-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-020-00460-7
This article is cited by
-
Real-time fluorescent detection of food spoilage with doped quantum dots-anchored hydrogel sensor
Nano Research (2024)
-
Sample-to-answer platform for the clinical evaluation of COVID-19 using a deep learning-assisted smartphone-based assay
Nature Communications (2023)
-
A portable immunosensor provides sensitive and rapid detection of Borrelia burgdorferi antigen in spiked blood
Scientific Reports (2023)
-
Smartphone-based hand-held polarized light microscope for on-site pharmaceutical crystallinity characterization
Analytical and Bioanalytical Chemistry (2023)
-
Smartphone-based paper microfluidic competitive immunoassay for the detection of α-amanitin from mushrooms
Microchimica Acta (2022)



